Emission performance and drive cycle measurements for diesel combustion engine passenger cars have created a lot of “smoke” in the media lately. The discussions highlight the importance of emission reduction and performance, and also reveal the great challenge of this work. If some of the largest car manufacturers in the world struggle to meet the emission limits, it is evident that there is not an easy solution.
Emissions are by-products of a chemical process, and to understand, predict and design compliant vehicles, the underlying chemical pathways need to be well understood. The purpose of this article is to introduce the tools available to make the job understanding and predicting chemistry easier and more straightforward. Here is a story about Jane, an imaginary combustion chamber design engineer, which describes the daily challenges she faces.
Meet Jane, a combustion engineer and STAR-CCM+ user
Jane is a newly employed design engineer at CombustionCorp, a company that just started to use CFD for a major part of their development projects, the design of a combustion chamber being one of them. Jane’s first assignment is to investigate and improve the design of a natural gas combustor. Using STAR-CCM+®, she is able to set up the geometry and the physics, opting for the standard Eddy Break Up (EBU) combustion model. She decides that keeping it simple by just modeling one overall reaction is a good starting point as she is under a lot of pressure from her management to get the job done quickly. She obtains a flow and temperature field and verifies that the results obtained are accurate. Next, she starts her design work with Optimate+TM, and she is able to automatically improve the design by optimizing the fuel nozzle placement.
And then, a few days later, a scientist from the lab tells her the combustor she is designing behaves differently for different qualities of natural gas. She wonders if she needs to consider a more complex approach and starts asking questions…
Why Does the Combustor Behave Differently for Different Natural Gas Qualities?
Different natural gas qualities contain varying amounts of larger hydrocarbons. High quality natural gas consists mainly of methane, while low quality natural gas includes a few percent of ethane, propane and butane. Larger hydrocarbons break much more easily than methane, and hence low quality natural gas ignites faster.
How Does the Early Ignition Affect the Overall Combustion Behavior?
To understand this, Jane studies the chemical effects in an isolated environment, eliminating the influence of flow fields. She finds she can use DARS to accomplish this. DARS is a standalone tool from CD-adapco for analyses of chemical reactions in 0D and 1D idealized reactors. The tool can read and analyze chemical reaction schemes, for example for hydrocarbon combustion and catalytic processes in after-treatment systems.
Jane opens DARS and reads in the standard natural gas chemistry delivered with DARS. Then she connects a few different reactors to the read mechanism module:
- Freely Propagating Flame: This module calculates the laminar flame speed, which is an important property for the flame propagation and thus the combustion behavior in a combustor. The module also calculates species profiles and temperature in the flame.
- Constant Pressure: This module calculates ignition delay times as well as species profiles and thermodynamic properties of an auto-ignition event under homogeneous constant pressure conditions. It can also be used to calculate emission production.
- Flamelet Library: This module calculates the species and temperature profiles in a diffusion flame. It also calculates the extinction limit.
- Equilibrium: This module calculates equilibrium species and temperature.
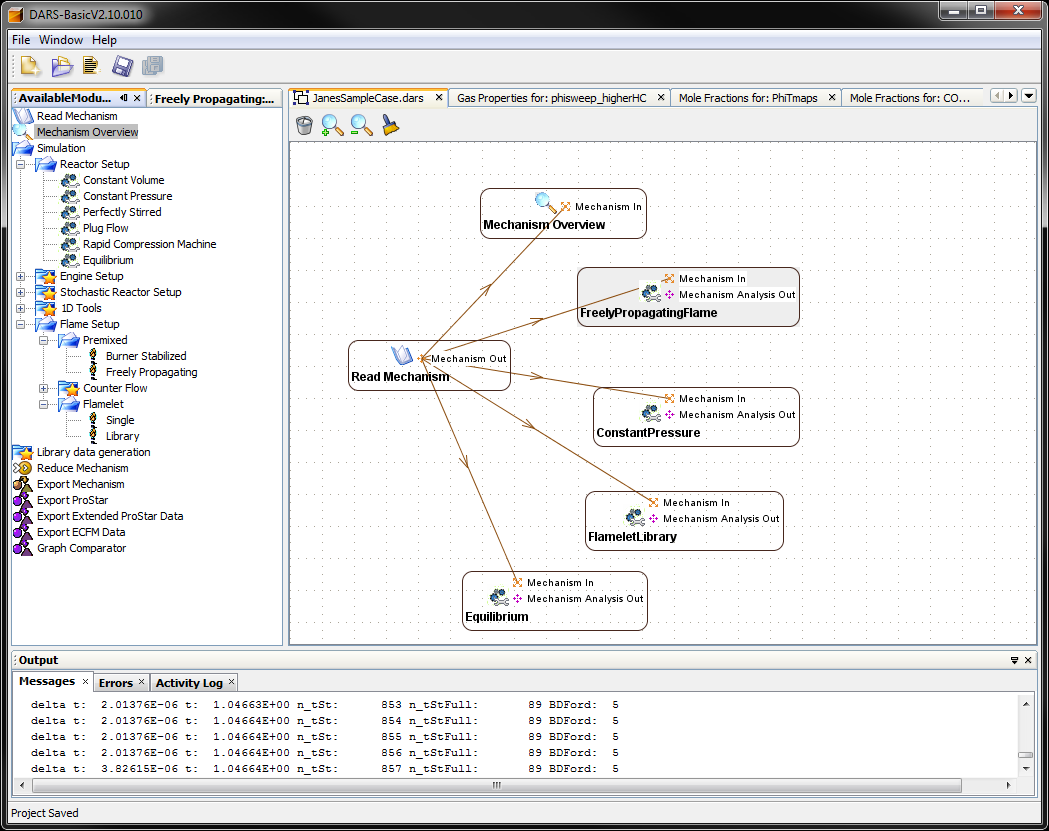
For each module, Jane tries two different fuel qualities in DARS:
- Pure methane to simulate very high quality natural gas,
- Methane mixed with a few percent of larger hydrocarbons (C2-C4).
She first calculates the laminar flame speed for a range of equivalence ratios. The laminar flame speed is the speed of a freely propagating flame under premixed conditions. She finds that the flame speed is about 1 cm/sec faster for the low quality blend. She contemplates if this matters for her design…
What Does a Faster Flame Speed Mean for the Combustor?
Jane decides that this increases the risk of flashback in the combustor. She also notes that under very lean conditions, the pure methane fuel speed is about 14% slower than the lower quality fuel, indicating the high quality natural gas is more prone to lean blow-off.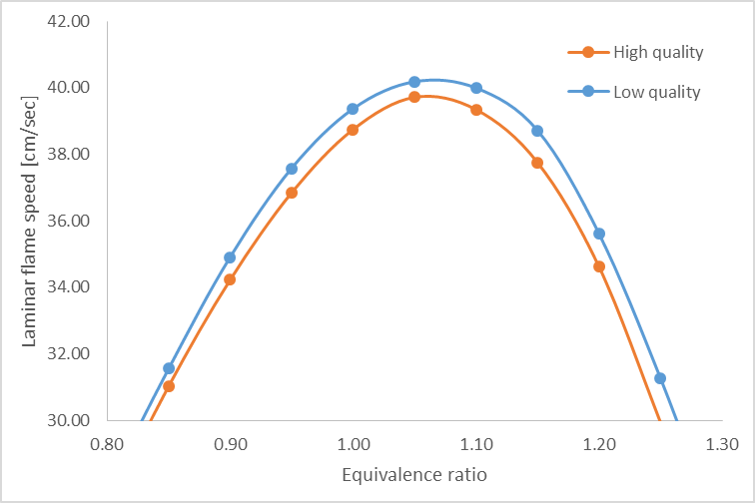
To understand the behavior under diffusion combustion, she calculates a flamelet library for each fuel composition. A flamelet is an idealized laminar diffusion flame, and a flamelet library is a set of flamelets for various scalar dissipation (mixing) rates. She observes that the maximum temperature in the flamelet is about 30K higher for the low quality fuel under high scalar dissipation rate. This makes the high quality natural gas flame more prone to extinction; she notes that the extinction scalar dissipation rate is 41/s for the low quality fuel, and 35/s for the high quality fuel. The extinction scalar dissipation rate is the mixing rate at which the diffusion flame is blown out. This indicates that blow-out is more common for high quality natural gas.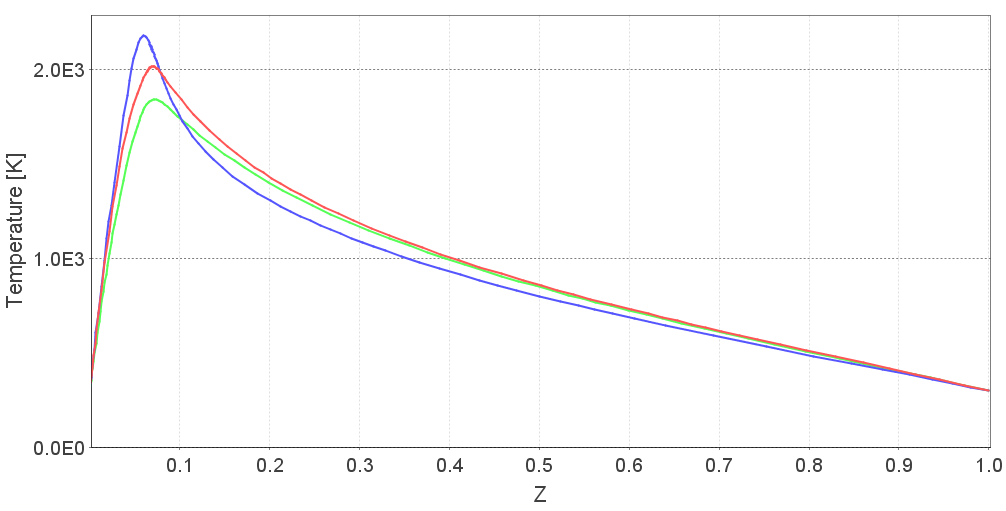
Jane calculates ignition delay times to understand the ignitability and to evaluate the tendency of pre-ignition in the mixing zone of the combustor. She creates a parameter sweep (called multi-run in DARS) with methane as fuel, sweeping over the full range of fuel-air equivalence ratios given the inlet temperature and ambient pressure. After a few seconds, the calculations are finished, and she can watch the ignition of the mixture. She notes that the time-to-ignition is shortened by about 25% for low quality natural gas, which gives an increased risk for pre-ignition in the mixing zone.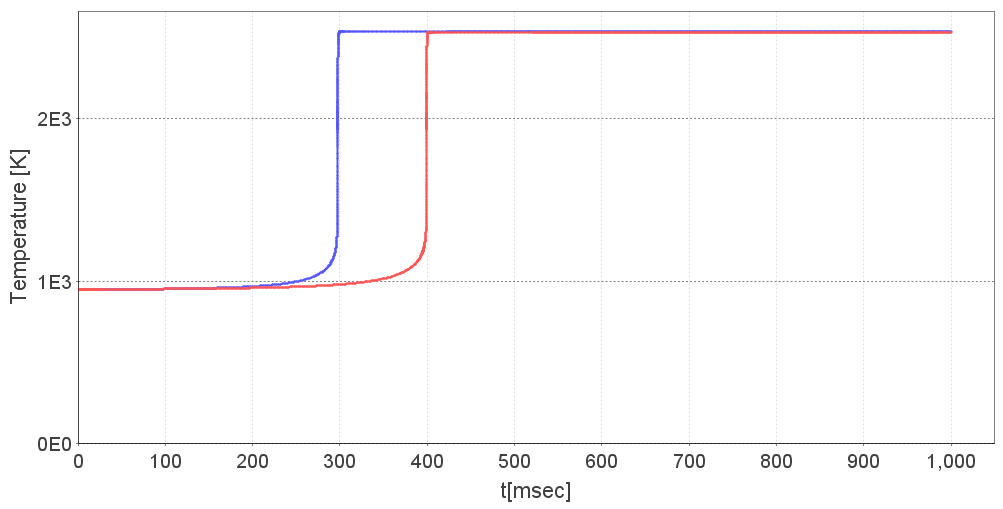
From the equilibrium calculations, she observes that the adiabatic flame temperature for low quality natural gas is about 5K higher than for high quality natural gas. Seeing the effect on the combustion behavior with only a very slight change in fuel composition, she understands she needs to continue the studies in the CFD simulations to quantify the effect on the combustor behavior. She now needs to figure out how to do this and wonders…
How Do I Account for Different Fuel Blends in my CFD Calculations?
Jane figures out that she can do this by using the Flamelet Generated Manifold (FGM) model in STAR-CCM+, which includes the full detailed finite rate chemistry without compromising the speed of execution. The effect of different fuel blends is accounted for through the creation of one FGM library for each fuel blend. The FGM library is generated from detailed chemistry.
How Do I get an FGM Library for my Combustor Conditions for STAR-CCM+?
Jane finds out that she can use DARS for this as well. She opens her DARS project and drags an FGM library generation module to the workbench, sets up the calculations and runs the library generation. She creates a set of FGM libraries for varying natural gas mixtures and uses them in her CFD calculations, adding different fuel qualities to her Optimate+ optimizations. She finds a design which is also suitable for low quality natural gas and delivers it to the lab engineers for testing.
After this update, her colleagues are very interested in the varying behavior and want to understand why this happens.
How to Understand the Effect of Varying Fuel Mixtures?
To do this, she re-runs the homogeneous constant pressure reactor case with low quality natural gas and methane only, and checks the species sensitivity analysis. The results for pure methane combustion look as follows: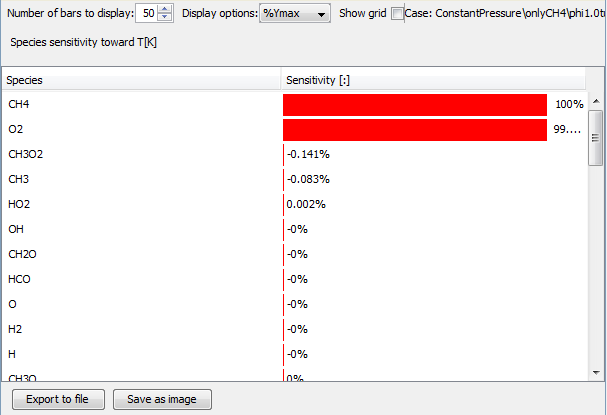
As expected, methane and oxygen are the dominant species for the combustion process. Then she plots the same sensitivity analysis for the low quality natural gas: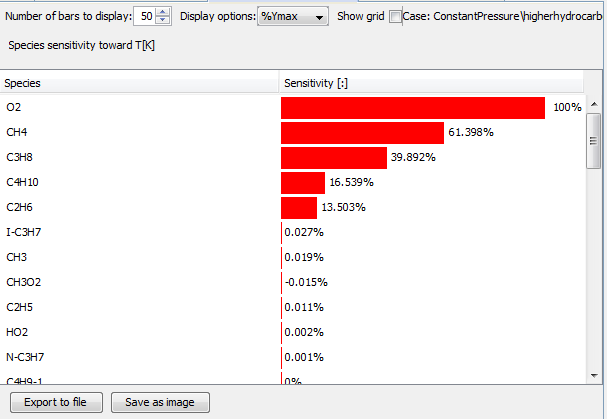
Propane constitutes only 1.5% of the fuel blend, but still affects the combustion almost as much as methane. Butane, which constitutes only 0.3% of the mixture, also has a significant effect on the combustion. This shows that the larger hydrocarbons have a large impact. To understand the reactions behind this behavior, she checks their sensitivities. She notices that propane and butane dissociation are two very important processes, in addition to the oxidation reactions: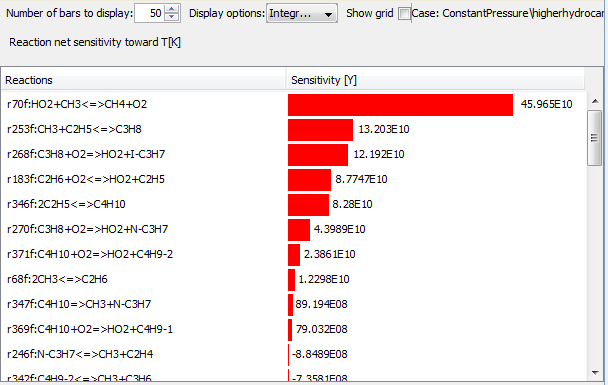
Using the sensitivity analyses, plots on ignition delay times and the CFD simulations, Jane is now armed with all the material she needs to explain to her colleagues what happens during this process.
A while later, the lab engineer returns and asks her about emissions. For some load points, the CO emissions are unacceptably high. Is she ready for her next challenge?
How to Improve Emission Performance?
To better understand the CO emissions, Jane starts up DARS and runs a set of homogeneous constant pressure reactors under constant temperature, creating an emission map: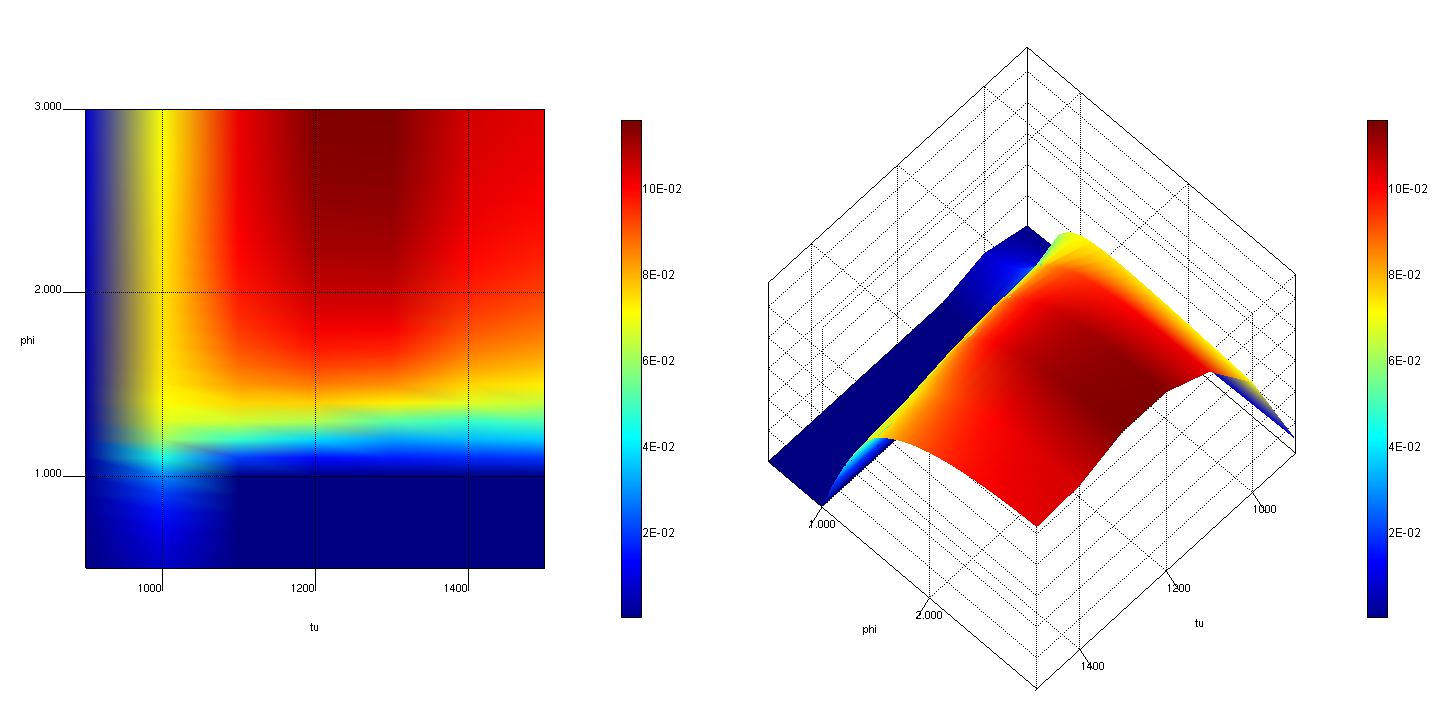
In this map, she can see the CO production for different fuel-air equivalence ratios and different temperatures. Comparing to the mixture fraction and temperature flow field in CFD, she finds her combustor entering the CO yield area in some fuel rich zones close to the fuel outlet for the load range specified by her colleague. She needs to improve the mixing in these zones, and thus sets a constraint on the maximum equivalence ratio in these regions for the next optimization loop. She manages to decrease the CO with some efficiency penalty, and can study the trade-off between CO emission and efficiency. To further understand the CO yield, she adds CO to the post-processing species in her FGM library generation, and re-generates the FGM libraries. Now she can directly study the CO yield in her CFD simulation.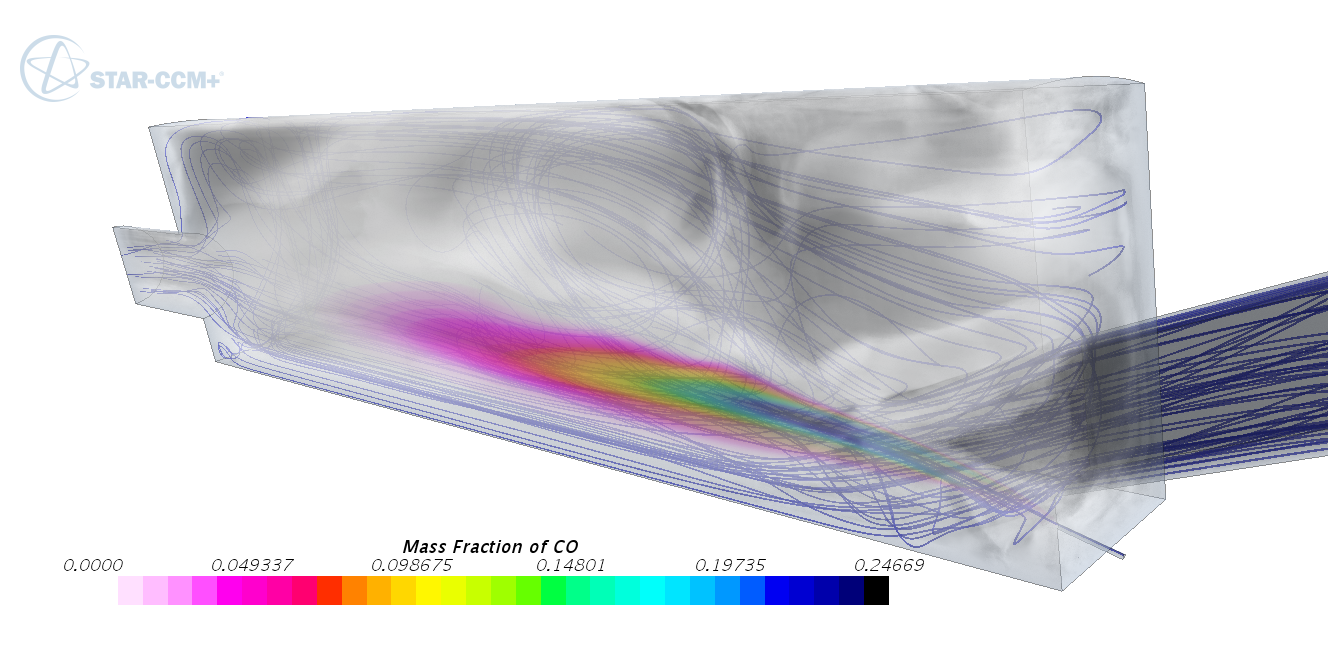
Finally, to fully understand the effects of detailed chemistry, she picks one of the cases and applies Complex Chemistry in STAR-CCM+ to perform a full CFD simulation with the chemical mechanism used in DARS. This serves as a good benchmark against the other combustion models. She convinces her manager that although these simulations take longer to complete, they will provide much better accuracy. After all, the company doesn’t want to be pulled over for non-compliance. Longer computational time might just be a small price to pay.
In addition to performing the complex chemistry calculations described in this article, DARS can be used to:
- Generate combustion and emission libraries for in-cylinder combustion:
- ECFM-3Z TKI
- ECFM-CLEH TKI + Equilibrium
- PVM-MF
- Soot
- Calculate laminar flame thickness
- Calculate surface and gas phase chemistry in catalysts (DOC, TWC, DPF, …)
- Reduce mechanisms
Source: CD-adapco